A research team led by Professor Jinah Jang (ME, LIFE, CiTE, GSCST) has successfully developed a 3D gastric cancer model capable of accurately predicting drug responses in cancer patients. This achievement was made through collaborative research with Prof. Charles Lee’s team at The Jackson Laboratory for Genomic Medicine in the United States. This study, while preserving the characteristics of patient tissues, evaluates individual drug responses and suggests the potential for predictive applications. This research was published in the international journal Advanced Science.
One of the most significant challenges in cancer treatment is “tumor heterogeneity.” Just as the taste of a dish can vary despite following the same recipe, cancer tumors exhibit different characteristics in each patient, leading to diverse drug responses. Current methods to predict drug efficacy include the Patient-Derived Xenograft (PDX) model, where patient-derived tumor tissues are implanted into immunodeficient mice to study cancer growth and drug responses, as well as genomic analysis of cancer cells. However, these approaches are time-consuming, costly, and not applicable to all patients.
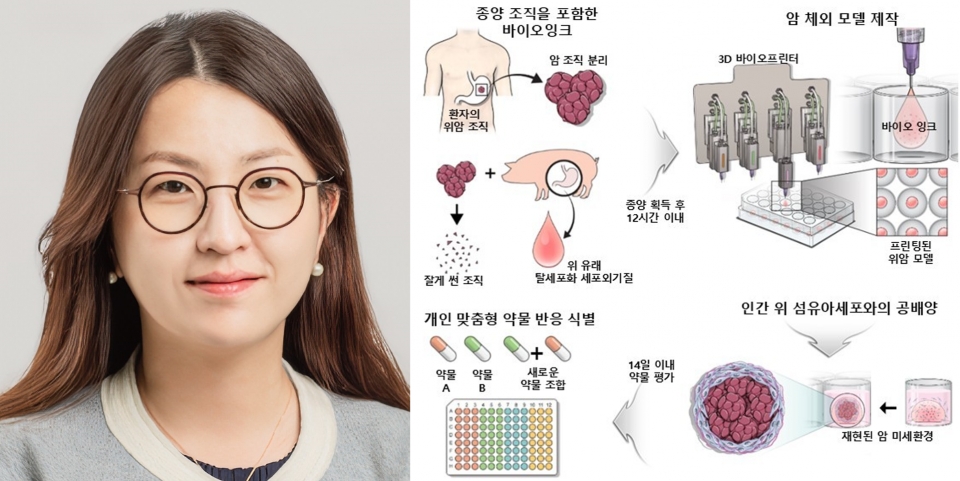
To address these limitations, the research team employed 3D bioprinting technology. The team created bioinks using patient-derived gastric cancer tissue fragments and a decellularized extracellular matrix (ECM) hydrogel derived from the stomach. By co-cultivating gastric fibroblasts, the researchers successfully replicated the tumor microenvironment with high precision.
Compared to the conventional PDX model, the research team’s 3D gastric cancer model exhibited gene expression patterns related to cancer initiation, growth, and drug responses that more closely resembled those in actual patients while preserving patient-specific gastric tissue characteristics. It also demonstrated higher accuracy in predicting anticancer drug efficacy and patient prognoses. Notably, this model allows for rapid drug response evaluation within two weeks of tissue collection.
This research was supported by the University-Centered Research Laboratory Support Program of the National Research Foundation of Korea, the Ministry of Science and ICT, and the STEAM Research Initiative, including the Future-Convergent Technology Pioneer and Mid-Career Researcher Support Programs.


