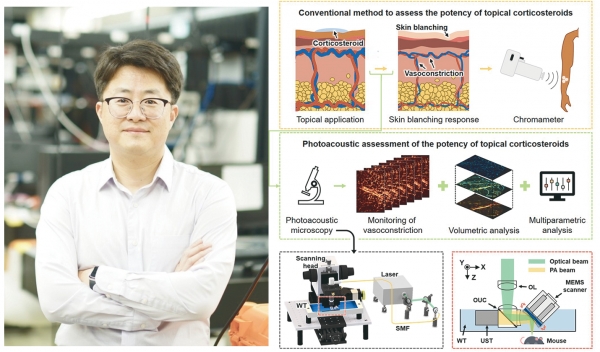
A research team led by Professor Chulhong Kim (EE, CiTE, IBIO, and ME) at POSTECH, along with Donggyu Kim (CiTE Ph.D. student) developed a technology in collaboration with the U.S. Food and Drug Administration (FDA) to visualize vasoconstriction induced by corticosteroids in mouse skin using photoacoustic microscopy (PAM). This research was published in the international journal Photoacoustics.
Steroid-based drugs are widely used to treat various skin conditions such as dermatitis, allergies, and autoimmune diseases. In particular, topical corticosteroids are effective in reducing inflammation and redness, but their precise mechanisms of action have been challenging to understand. Traditional methods using a chromameter to measure changes in skin surface color only provide indirect assessments of the drug’s effects, making it difficult to observe internal changes such as vasoconstriction directly.
In this study, the joint research team from POSTECH and the U.S. FDA successfully implemented non-invasive 3D imaging of vascular changes in the mouse skin using PAM technology, which combines light and sound. This technique allows for safe and precise observations without the need for ionizing radiation, such as X-rays, or additional drugs, thus minimizing the burden on the human body.
Using PAM technology, the research team quantitatively compared and analyzed the vasoconstriction capabilities of four different topical corticosteroids and elucidated the mechanism of vasoconstriction concerning skin depth through 3D imaging. The analysis results aligned with the National Psoriasis Foundation’s classification of corticosteroid potency, demonstrating the reliability and utility of PAM technology.
Prof. Kim from POSTECH commented, “This study presents a new non-invasive method for evaluating drugs, which will greatly aid clinical research and bioequivalence assessments. Through collaboration with the FDA, we have also confirmed the potential of PAM technology in studying the side effects of skin disease treatments.”
This research was supported by the Ministry of Education, the Ministry of Science and ICT, and the Medical Device Development Fund project.


