Professor Si-Young Choi (MSE), So-Yeon Kim (Integrated program, MSE), and Yu-Jeong Yang (Integrated Course, MSE) of POSTECH have recently succeeded in identifying the surface structure stabilization mechanism of high-Ni cathodes material through single-element doping, in joint research with Dr. Sungho Choi of Korea Research Institute of Chemical Technology (KRICT), and Sora Lee and Chiho Jo of LG Energy Solution. The research has been published in the international academic journal Chemical Engineering Journal.
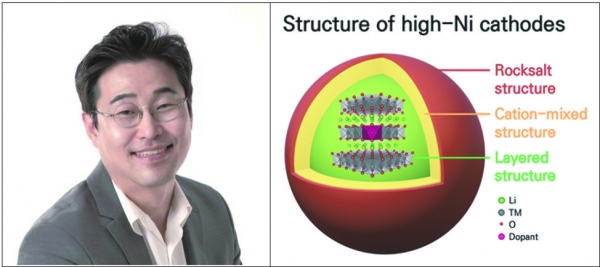
In electric vehicles, cathode materials with high capacity, such as high-Ni cathodes, are needed to enable batteries to store higher electric power. On the cathodes’ surface, however, nickel and lithium particles of similar sizes switch positions as the content of nickel increases. This could lower the capacity of the batteries if the intermixing of cations occurs excessively.
To address this issue, recent research has been focusing on adding metal ions as a dopant to the lithium layer or the transition metal layer of cathode material. Identifying the proper doping site is critical for discovering the effect cation has on the structure stabilization mechanism. Unfortunately, the usage of these dopants is limited to small amounts, which impedes the identification of accurate doping sites.
The research team developed deep-learning AI technology that performs quantitative analysis of cation mixing and combined it with atomic-scale electron microscopy technology. The team also visualized the doping position of different dopants (Al, Ti, Zr) with limited concentrations, and analyzed its effect on cathode material. The result showed that the doping on the transition metal layer strengthened the bonding between nickel and oxygen atoms and enhanced structural stability by constraining cation mixing.
The analysis was a cornerstone of quantitative analysis of cation mixing, which previously had only been analyzed qualitatively. Prof. Choi hopes to contribute to “identifying mechanisms for enhancing the performance of cathode material by establishing a foundation for analyzing high-sensitive materials.”
The research was supported by the Ministry of Science and ICT (MSIT), Institute of Civil Military Technology Cooperation, Ministry of Education (MOE), and LG Energy Solution.


