The research team of Professor Jong Kyoung Kim (LIFE) and Integrated Ph.D Candidate Yujin Jeong (LIFE) revealed the mechanism that regulates the inflammation and metabolic dysfunction triggered by obesity through joint research with teams of Prof. Yun-Hee Lee (SNU Pharmacy), Prof. Young-Min Hyun (YU Medicine), Prof. Granneman (WSU), and Prof. Young-Suk Jung (PNU Pharmacy). The research was published in the international journal, Nature Communications.
According to the World Health Organization (WHO), the obese population took up 16% of the whole population in 2022. Obesity is one of the fastest-growing diseases in the world and is assumed to be the key cause of many metabolic diseases such as diabetes, high blood pressure, and atherosclerosis.
During overnutrition, immune cells are recruited to the adipose tissue (AT). Some of the immune cells support healthy tissue homeostasis by removing apoptotic (“dead”) cells, whereas others are proinflammatory immune cells that induce inflammations. In the case of obese patients, the latter proliferate (multiply) rapidly causing serious problems in inflammatory response and metabolism.
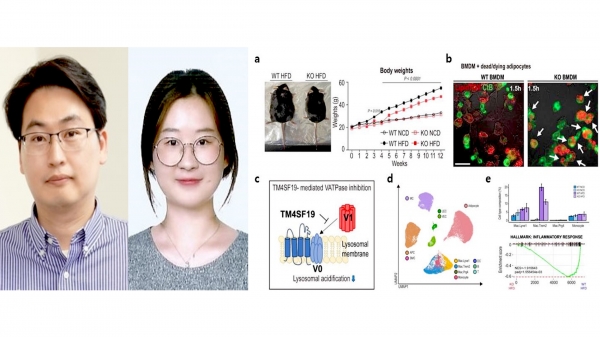
The research team used animal experiments, single nucleus RNA sequencing, and intravital imaging to analyze transmembrane 4 L six family member 19 (TM4SF19) proteins that are expressed by inflammatory macrophages.
The research results showed that TM4SF19 increased in the AT of mice fed a high-fat diet (HFD). The team revealed for the first time that this protein blocks the vacuolar H + -ATPase (V-ATPase) pump of the lysosome, affecting lysosomal acidification and thus resulting in stalled efferocytosis, the mechanism of removing apoptotic cells.
TM4SF19 deletion improved efferocytosis of macrophages, reduced weight gain of HFD mice and prevented inflammatory response and insulin resistance of AT, improving metabolic dysfunction. The research is significant in the sense that the research team discovered that TM4SF19 was the key factor to the solution for relieving obesity-induced inflammation and metabolic dysfunction. Prof. Kim stated that “we finally found the mechanism of TM4SF19-mediated control of lysosomal activity.”, anticipating that “the research will shed new light on the potential therapeutic of metabolic diseases including obesity.”
The research was funded by the National Research Foundation of Korea (NRF), the Korea Mouse Phenotyping Center (KMPC), Korea University Medicine, and the National Institutes of Health (NIH).


