One of the key elements in the industrial sector is NH3, ammonia. For its high hydrogen content and energy density, ammonia serves as a carrier for storing and transporting hydrogen. Researchers have increased the efficiency of ammonia production by using sulfur (S).
The current method for producing ammonia is the Haber-Bosch process, which, however, requires high temperatures (400-500 °C) and high pressure (200-300 atm). Due to these conditions, the process consumes a significant amount of energy. Which leads to a significant environmental burden.
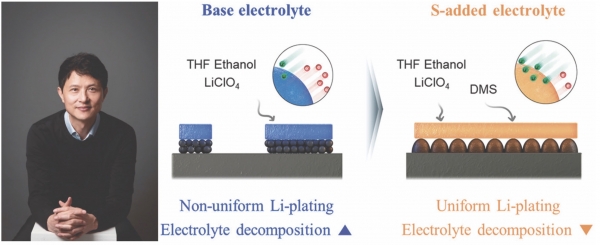
Recently, the use of lithium (Li) in the Nitrogen Reduction Reaction (Li NRR) has emerged as an alternative. The abundant nitrogen gas (Nâ‚‚) in the atmosphere, with its two nitrogen atoms bonded by a triple bond, can be effectively reduced to produce ammonia by plating reactive lithium on the electrode, breaking the strong bond. While the method is environmentally friendly, its stability and production efficiency have been relatively low. To enhance efficiency, it is essential to uniformly deposit lithium on the electrode and improve the stability of the Solid Electrolyte Interphase (SEI) at the interface of the electrode.
In this recent study, the research team led by Professor Kijung Yong (CE) utilized sulfur for the reaction. When sulfur is used in minute quantities, it enhances reaction activity. The addition of sulfur-containing dimethyl sulfide led to the formation of lithium sulfate
(Liâ‚‚SO4) and lithium sulfide (Liâ‚‚S) molecules, facilitating smooth lithium ion movement. Consequently, lithium was uniformly deposited on the electrode, and the Solid Electrolyte Interphase (SEI) transformed from a dense and thin film structure to a mesh structure. This structure increased ion conductivity, allowing lithium to deposit more uniformly.
Furthermore, sulfur prevents the decomposition and degradation of the SEI, enhancing the stability of the process. Experimental results showed that cells from the research team with added sulfur exhibited over twice the energy efficiency of conventional batteries even after more than 20 hours of use. Prof. Yong expressed, “This research, which enables more efficient production of ammonia, will significantly contribute to advancing eco-friendly hydrogen technology.”
Meanwhile, this research was published in the ACS Energy Letters. The research was conducted with support from the National Research Foundation (NRF) funded by the Korean government (MSIT). Also Prof. Ib Chorkendorff’s research group at DTU provided much advice in conducting this study.


